I first blogged about wood packaging in July 2015 – it was my first blog! I have written 15 times about wood packaging since. To see the series, visit www.nivemnic.us, scroll down below “archives” to “categories”, click on “wood packaging”.
For five years, I have called upon USDA to act. It’s long past time to replace decade-old policies that have failed to prevent introductions. More recently, I have begun calling for revising the international phytosanitary system, too.
As I’ve demonstrated in my blogs – and documented by Aukema et al. 2011 and others—wood-boring beetles have been among the most damaging tree-killing pests introduced to the U.S. Local governments, homeowners, and businesses spend billions of dollars each year to manage dying and dead trees. Landowners bear added costs in reduced property values. The ecosystem impacts are substantial, but still poorly quantified.
International efforts – i.e., ISPM#15 – have apparently reduced the rate at which wood-borer pests approach our shores. However, the reduction has not been sufficient to prevent a tripling of the number of non-native wood-borers established in U.S. by 2050 (Leung et al. 2014) — as I have demonstrated over and over.
Also, I have documented again and again the continued presence of wood-borers in incoming wood packaging and resulting introductions (visit the “wood packaging” category in the blog archives).
Part of the blame for inadequate protection from pests might arise from the specific requirements of current international standards (see Nadel et al. 2016 and Krishnankutty et al. 2020b). But I think most of the blame falls on APHIS’ choice to be forgiving, rather than strict, in enforcing its own regulations that implement the international standard.
There is widespread evidence of exporters’ failures to implement international standards. The evidence is clear: we cannot rely on exporters to meet either international standards or importing country’s phytosanitary requirements. The same countries – and even individual exporting businesses! – fail to comply with ISPM#15 year after year (Haack et al. 2014; APHIS interception database). APHIS has not taken effective action to end imports from these scofflaws.
U.S. phytosanitary policy is set by politicians. Politicians pay more attention to constituents’ concerns during election seasons – so NOW is the time to press for changes! I will discuss how to do this in an accompanying second blog.

U.S. imports have decreased significantly in recent years, especially from the two countries with the worst records of non-compliance with ISPM#15 (Mexico and China). But economic collapse is not a long-term strategy for reducing pest risk.
Quantifying Pest Risk for Wood Packaging: We Don’t Know
Here’s my best estimate of the pest risk associated with wood packaging. Remember, though, that key data remain missing.
Haack et al. published a landmark analysis of pest approach rates in 2014, using data from 2009. However, they did not include imports from China, Mexico, or Canada. Given the history of interceptions, it is probable that a recalculation of the approach rate that included China and Mexico would raise the estimate. It is more difficult to provide a more accurate estimate re: Canada, because CBP rarely inspects those shipments. (The U.S. and Canada do not require each other to treat wood packaging.)
As of mid-October 2019, CBP said it received 11 million containers at seaports annually (CBP website). If 75% of those incoming sea-borne containers have wood packaging (per Meissner et al. 2009), that equals 8,250,000 containers. If 0.1% of those containers with wood packaging is infested (per Haack et al. 2014), we are receiving 8,250 infested shipping containers via maritime shipping – even now, when imports have decreased substantially. This is more than 22 infested containers every day.

As of a decade ago, Chinese shipments were only half as likely to be enclosed in wood packaging as are shipments from other exporters. Perhaps this reflects a greater reliance on air shipments – air shipments globally are half as likely as maritime shipments to be encased in packaging made of wood (Meissner et al. 2009). Despite the lower proportion of wood packaging use, shipments from China still rank second (to Mexico) in the number of shipments detected as infested. In part, the data reflect inspection priorities: due to the great damage caused by Asian insects to North American trees and their record of poor compliance, CBP targets shipments from China for more intense scrutiny. Still, the high number of detections reflects continuing non-compliance by Chinese exporters. And remember: first, the U.S. and Canada began requiring treatment of wood packaging from China at the end of 1998 – 21 years ago! And second, APHIS almost never penalizes importers for poor compliance.
Understanding the pest risk from Mexico and Canada is important, because they are our second and third largest trading partners. As of October 2019, the numbers of shipping containers arriving overland (by truck or rail) from these countries annually were 13.7 million (CBP website). No one has estimated the proportion of these containers that contain wood packaging. If it is the same proportion as in maritime shipments, the approach rate would be another 10,275 infested containers per year – or 28 per day.
The total of maritime and land-based shipments that are probably infested (excluding air shipments) – would be 18,525 containers annually or 50 per day.
If I am right that shipments from China and Mexico have a higher pest-infestation rate than the 0.1% global estimate developed by Haack et al. (2014), the pest approach rate is probably higher than the 18,525 containers given above.
(I noted in my previous blog that insect species arriving from our neighbors pose a lower risk than the species from Asia or Europe – although the risk is not “0”. I addressed the Mexican woodborers in the previous blog. The risk from Canada could arise from non-native woodborers established in that country but not yet in the U.S. e.g., brown spruce longhorned beetle. Another risk is that shipments from off-shore origins might be transshipped through Canada and escape inspection because they are claimed to have been re-packaged there – as CBP staff have told me.)
The point is, we don’t know how many pests are reaching the U.S. daily. The current approach rate might be significantly higher or lower than Haack and colleagues estimated a decade ago due to
- Exclusion of China, Mexico, and Canada from the original study.
- Changes in the treatment requirements of ISPM#15.
- Another decade of experience – which might have led to better compliance (however, see below).
Despite my urging, APHIS has not agreed to a study to update Haack’s estimate.
It is also true that shipping containers provide shelter for a vast range of hitchhiking organisms in addition to insects in the wood, e.g., other insects’ eggs attached to the sides of the container, snails, weed seeds, even vertebrates.
Enforcement: One Agency Steps Up
When ISPM#15 was adopted, APHIS expected that importers would clean up their supply chains in order to avoid the lost income and costly delays that result from CBP interception of a non-compliant shipment. However, the data clearly show that this disincentive to violate ISPM#15 is insufficient to prompt companies to fix the problem. We need to find a more efficacious approach.
Clearly, enforcement in the form of penalties had been rare before 2017. CBP staff reported that as of January 2017 (before the agency strengthened its own enforcement effort), only about 30 of the nearly 21,000 non-compliant import shipments had received a financial penalty. CBP staff cited two reasons for the low penalty rate: 1) USDA policy requires that an importer be caught five times in a year with non-compliant wood packaging before imposing a fine; and 2) APHIS had not designated wood packaging as a high-risk commodity. After CBP initiated more aggressive enforcement in November 2017, enforcement actions rose by 400% (John Sagle, CBP. pers. comm) – although from a very low starting point!
Data on CBP interceptions in 2019 (Harriger) show decreases in the number of non-compliant shipments from earlier years in all categories: a 19% decrease below the 2010-2018 average of shipments intercepted; a 13% decrease in number of shipments intercepted because the wood packaging lacked the ISPM#15 mark; a decrease of 6% in the number of shipments intercepted that had a quarantine pest. Still, percentages based on absolute numbers don’t tell the whole story. They can be affected by inspection effort and other variables. So while these decreases are encouraging, it is still too early to determine the impact of CBP’s enforcement upgrade.
Unfortunately, there has not yet been the substantive/overall change needed in federal policy. At a minimum, APHIS continues to allow importers five violations per twelve month period.
While the cities that import the most goods – especially from Asia – would seem to be at particular risk, experience shows that pests can be introduced anywhere. This is demonstrated by establishment of the Asian longhorned beetle in semi-rural Clermont County, Ohio and the velvet longhorned beetle in Utah (Krishnankutty, et al. 2020a).

“Treated” Wood Still Transports Pests
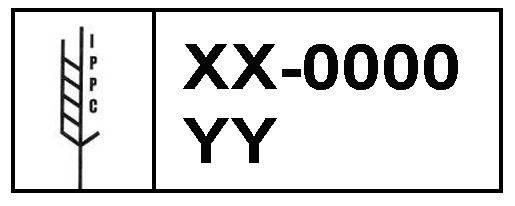
According to interception data provided to me by CBP (Harriger), 97% of pest-infested shipments detected over a period of 6 years (FYs 2010 – 2015) bore the stamp indicating they’d been treated in compliance with ISPM#15. These shipments came from all importing countries. Unfortunately, CBP has not provided the necessary breakdown of its data in more recent years to calculate this proportion.
Krishnankutty et al. (2020b) analyzed wood packaging from 42 countries intercepted by CBP over six years (April 2012 – January 2018). They found that 87% of the infested wood packaging included in this study bore the ISPM mark. This is a lower non-compliance rate than that shown by CBP data, but still too high.
European scientists carried out an intensive survey of wood packaging associated with shipments of stone from China to the 28 European Union countries during 2013-2016. They also found that 97.5% of consignments that harbored pests bore the ISPM#15 mark (Eyre et al. 2018). The problem did not decrease over time.
The possible causes of this problem are long-known. What effort is APHIS making to determine their relative importance? Is it fraud? Is it accidental misapplication of the treatments? Is it that the treatments do not work as well as necessary?
By comparing Haack’s estimate with the CBP data, I estimate that CBP is detecting and halting the importation of 4 – 8% of the shipments that actually contain pest-infested wood. Wu et al. (2020) concurred that the majority of infesting larvae would probably not be intercepted – despite CBP’s best efforts – and would be transported to the cargo’s intended destinations.

Since CBP inspects only about 2% of incoming shipments, this detection rate demonstrates the value of CBP’s program to target likely violators. It deserves praise. But it is obviously too low a “catch” rate to provide an adequate level of protection for our forests. I do not believe that increasing the inspection workforce and effort will result in substantial improvement in this rate. Instead, we need action to curtail imports of wood packaging from countries and exporters with records of non-compliance.
What Federal Agencies Are Doing to Better Prevent Introductions
Other than CBP’s welcome newly rigorous enforcement policy, most actions have focused on educating exporters, importers, shippers, customs brokers, and exporting countries’ phytosanitary agencies.
Since upgrading its enforcement actions, CBP has expanded its long-standing educational efforts. APHIS co-sponsored workshops for agricultural agencies and exporters in Asia and the Americas earlier in the decade.
APHIS also planned to host international symposia on wood packaging issues as part of events recognizing 2020 as the International Year of Plant Health. These symposia have been postponed by travel and other restrictions arising from the coronavirus pandemic.
Posted by Faith Campbell
We welcome comments that supplement or correct factual information, suggest new approaches, or promote thoughtful consideration. We post comments that disagree with us — but not those we judge to be not civil or inflammatory.
For a detailed discussion of the policies and practices that have allowed these pests to enter and spread – and that do not promote effective restoration strategies – review the Fading Forests report at http://treeimprovement.utk.edu/FadingForests.htm
SOURCES
Aukema, J.E., B. Leung, K. Kovacs, C. Chivers, K. O. Britton, J. Englin, S.J. Frankel, R. G. Haight, T. P. Holmes, A. Liebhold, D.G. McCullough, B. Von Holle.. 2011. Economic Impacts of Non-Native Forest Insects in the Continental United States PLoS One September 2011 (Volume 6 Issue 9)
Eyre, D., R. Macarthur, R.A. Haack, Y. Lu, and H. Krehan. 2018. Variation in Inspection Efficacy by Member States of SWPM Entering EU. Journal of Economic Entomology, 111(2), 2018, 707–715)
Haack, R. A. 2006. Exotic bark- and wood-boring Coleoptera in the United States: recent establishments and interceptions. Can. J. For. Res. 36: 269–288.
Haack, R.A., F. Herard, J. Sun, J.J. Burgeon. 2009. Managing Invasive Populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: A Worldwide Perspective. Annu. Rev. Entomol. 2010. 55:521-46.
Haack, R. A., K. O. Britton, E. G. Brockerhoff, J. F. Cavey, L. J. Garrett, M. Kimberley, F. Lowenstein, A. Nuding, L. J. Olson, J. Turner, and K. N. Vasilaky. 2014. Effectiveness of the international phytosanitary standard ISPM no. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. Plos One 9:e96611.
Kevin Harriger, US CBP. Presentation to the annual meetings of the Continental Dialogue on Non-Native Forest Insects and Diseases, over appropriate years. See, e.g., https://continentalforestdialogue.org/continental-dialogue-meeting-november-2018/
Krishnankutty, S.M., K. Bigsby, J. Hastings, Y. Takeuchi, Y. Wu, S.W. Lingafelter, H. Nadel, S.W. Myers, and A.M. Ray. 2020a. Predicting Establishment Potential of an Invasive Wood-Boring Beetle, Trichoferus campestris (Coleoptera) in the United States. Annals of the Entomological Society of America, XX(X), 2020, 1–12
Krishnankutty, S., H. Nadel, A.M. Taylor, M.C. Wiemann, Y. Wu, S.W. Lingafelter, S.W. Myers, and A.M. Ray. 2020b. Identification of Tree Genera Used in the Construction of Solid Wood-Packaging Materials That Arrived at U.S. Ports Infested With Live Wood-Boring Insects. Commodity Treatment and Quarantine Entomology
Leung, B., M.R. Springborn, J.A. Turner, E.G. Brockerhoff. 2014. Pathway-level risk analysis: the net present value of an invasive species policy in the US. The Ecological Society of America. Frontiers of Ecology.org
Meissner, H., A. Lemay, C. Bertone, K. Schwartzburg, L. Ferguson, L. Newton. 2009. Evaluation of Pathways for Exotic Plant Pest Movement into and within the Greater Caribbean Region. Caribbean Invasive Species Working Group (CISWG) and USDA APHIS Plant Epidemiology and Risk Analysis Laboratory
Nadel, H. S. Meyers, J. Molongoski, Y. Wu, S. Lingafelter, A. Ray, S. Krishnankutty, A. Taylor. 2017. Identification of Port Interceptions in Wood Packing Material Cumulative Progress Report, April 2012 – June 2017
Oregon Department of Agriculture, Plant Protection & Conservation Programs. 2019. Annual Report 2019.
USDA APHIS interception database – pers. comm. January 2017.
U.S. Department of Transportation, Maritime Administration, U.S. Waterborne Foreign Container Trade by U.S. Customs Ports (2000 – 2017) Imports in Twenty-Foot Equivalent Units (TEUs) – Loaded Containers Only at https://ops.fhwa.dot.gov/freight/freight_analysis/nat_freight_stats/docs/06factsfigures/fig2_9.htm
U.S. Department of Transportation. Port Performance Freight Statistics in 2018 Annual Report to Congress 2019 https://rosap.ntl.bts.gov/view/dot/43525
Wu, Y., S.M. Krishnankutty, K.A. Vieira, B. Wang. 2020. Invasion of Trichoferus campestris (Coleoptera: Cerambycidae) into the United States characterized by high levels of genetic diversity and recurrent intros. Biological Invasions Volume 22, pages1309–1323(2020)