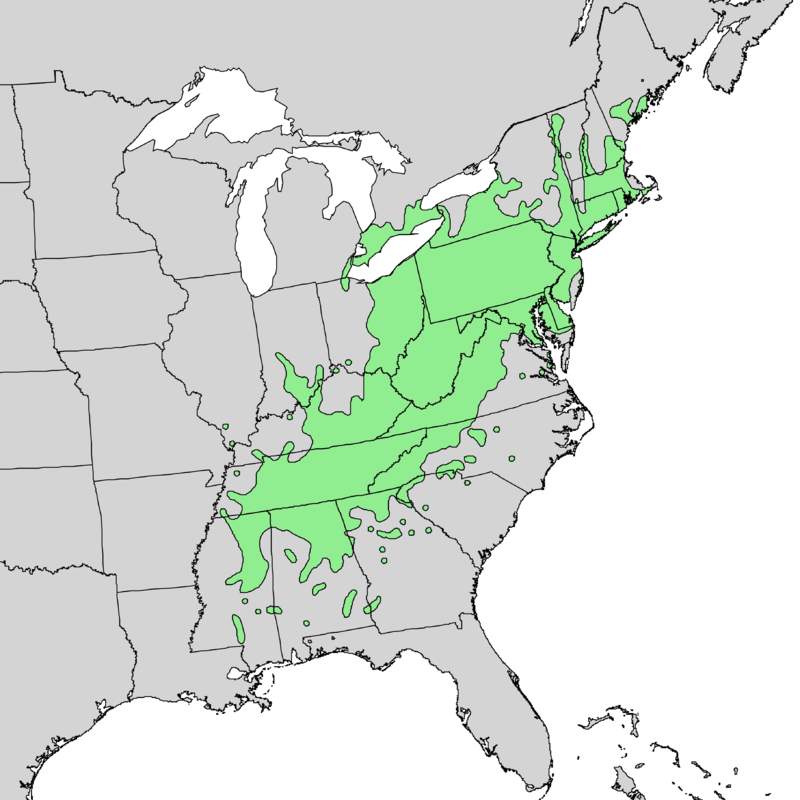
I participated in the annual USDA Interagency Invasive Species Research Forum in Annapolis in January 2023; as usual, I learned interesting developments. I focus here on updates re: efforts to protect ash and hemlock
Hopeful Developments re: countering EAB to protect ash
There are hopeful results in both the biocontrol and resistance breeding programs. The overall goal is to maintain ash as a viable part of the North American landscape.
Biocontrol
Juli Gould (APHIS) reminded us that the agency began a classical biocontrol program targetting emerald ash borer (EAB) in 2003 – only a year after EAB had been detected and much earlier than is the usual practice. [Thank you, former APHIS PPQ Deputy Administrator Ric Dunkle!] By 2007 scientists had identified, tested, and approved three agents; a fourth was approved in 2015.
Nicole Quinn (University of Florida) stressed that the egg prarasitoid, Oobius — if it is effective — could prevent EAB from damaging trees. However, it is so small that it is very difficult to sample. One small study demonstrated that Oobius will parasitize EAB eggs laid in white fringe trees (Chionanthus virginicus) as well as in ash. This is important because it means this secondary host is not likely to be a reservoir of EAB.
The numbers
According to Ben Slager (APHIS), more than 8 million parasitoids have been released at 950 sites since the program began in 2007. These releases have been in 418 counties in 31 states, DC, and four Canadian provinces. Still, these represent just 28% of infested counties. Parasitoids have been recovered in 21 states and two provinces.
Rafael de Andrade (University of Maryland) specified that these releases included more than 5 million Tetrastichus in 787 sites; ~2.5 million Oobius in 828 sites in 30 states; ~500,000 Spathius agrili – lately only north of the 40th parallel. Releases of Spathius galinae began in 2015; so far ~ 470,000 in 395 sites.
Impact
Several presenters addressed questions of whether the agents are establishing, dispersing, and – most important – improving ash survival. Also, can classical biocontrol be integrated with other management techniques, especially use of the pesticide emamectin benzoate.
Dispersal
Several studies have shown that the four biocontrol agents disperse well (with the caveat that Oobius is very difficult to detect so its status is much less certain).
Implementation considerations
De Andrade found that the longer the delay between the date when EAB was detected and release of Oobius, the less likely Oobius will be recovered. Tetrastichus surprised because the higher the numbers released, the fewer were recovered. He could determine no association between recovery of S. agrili and variations in release regime [numbers released; delay in releasing biocontrol agents; or frequency of releases]. He said it is too early to assess Sp. galinae since releases began only in 2015, but he did see expected relationship to propagule pressure – the more wasps released, the higher the number that were recovered. Sp. galinae did surprise in one way: it seemed to perform better at lower latitudes. De Andrade noted he was working data from less than half of release sites. He asked collaborators to submit data!!!!
Initial signs of ash persistence and recovery
Claire Rutledge (Connecticut Agriculture Experiment Station) determined that
- More large trees were surviving in plots where the biocontrol agents were released
- EAB density was lower at long-invaded sites
- Parasitism rates were similar across release age treatments and release/control plots
Gould focused on protecting saplings so they can grow into mature trees which could be sources of seeds to establish future generations. She noted that there are many “aftermath” forests across the northern United States – those dominated by ash saplings.
In Michigan, at a site of green ash, as of 2015 – 2021, EAB populations are still low, parasitism rate by Tetrastichus and S. galinae high. The percentage of saplings that remained healthy was greater than 80%. There were similar findings in white ash in New York: very low EAB larval density; and more than 70% of ash saplings had no fresh galleries. Gould reported that Tetrastrichus impcts could be detected within three years of release.
So, EAB are being killed by the biocontrol agents combined with woodpecker predation; but in their fourth instar, after considerable damage to the trees.

Jian Duan reported on two long-term studies in green & white ash in Michigan and New England. His team used the most labor-intensive but best approach to determine EAB larval mortality and the cause – debarking trees – to determine whether the EAB larva were parasitized, were preyed on by woodpeckers, or were killed by undetermined cause, such as tree resistance, disease, or competition. In Michigan, he linked a crash of EAB population in 2010 was caused by Tetrastichus; EAB tried to recover, but crashed again, due to S. galinae. EAB larval densities had been reduced to 10 / m2. Predation by abundant woodpeckers and the native parasitoid Atanycolus was also important.
In New England, EAB has also declined from 20-30 larvae /m2 to ~ 10 m2.
In Michigan, healthy ash with dbh of larger than 5 inches were much more plentiful in sites where parasitoids had been released. Their survival/healthy rate also was much higher in release sites but the difference declined as years passed. In New England there were growing numbers of healthy trees in 2021-22; (almost none in 2017). Duan conceded that he could not prove a direct link but the data points to recovery.
Tim Morris (SUNY-Syracuse) found that white ash saplings continued to die in large numbers, but the mortality rate was significantly below the rate in 2017. Canopy conditions varied; some trees that were declining in 2013 were recovering in 2017. Forty percent of “healthy” ash in 2013 continued recovering in 2021. Few living trees were declining; trees were either healthy or dead. He thinks probably a combination of genetics and presence of parasitoids explains which trees recover. Morris also reported some signs of regeneration.
At this point, I noted that in parts of northern Virginia, beavers have killed ash saplings. Morris reported finding the same in some sites in New York. Perhaps others have, also; my comment was greeted by laughter.
Theresa Murphy (APHIS) looked at integration of biocontrol and insecticide treatment in urban and natural sites. A study of black and green ash in Syracuse, NY Naperville, IL, and Boulder, CO found continued high parasitism by Tetrasticus and S. galinae and woodpecker attacks in trees treated with emamectin benzoate. Researchers could not detect Oobius. By 2020, most of the untreated trees had died but treated trees remained healthy.
Murphy has begun studying integration of biocontrol and pesticides in green and black ash forests. The goal is to protect large trees to ensure reproduction; the biocontrol agents do not yet protect the large trees. This is especially important for black ash because it declines very quickly after EAB invades. Sites have been established in New York, through collaboration with New York parks, Department of Environmental Conservation, and the Mohawk tribe. She is still looking for sites in Wisconsin – where EAB is spreading more slowly than expected.
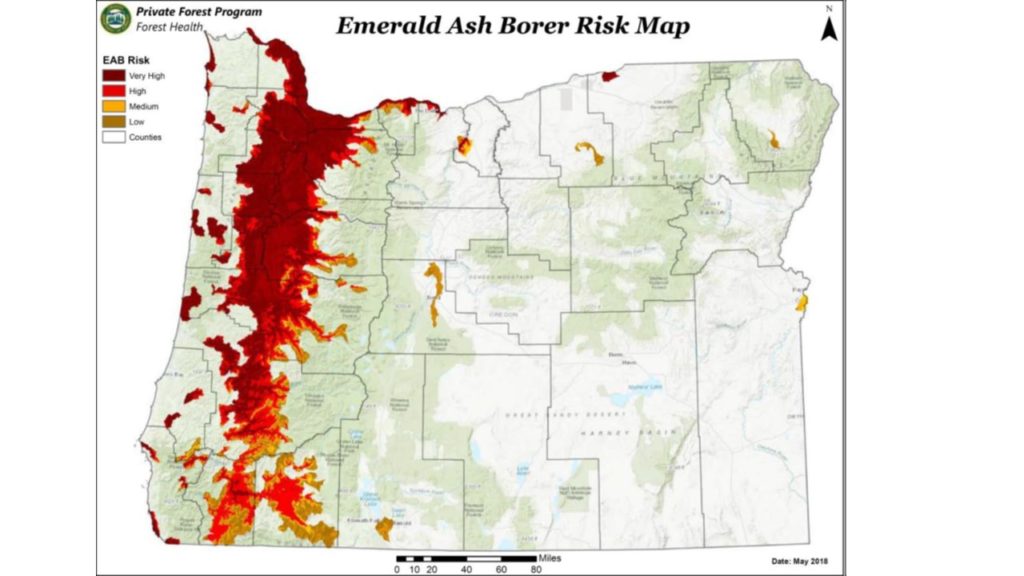
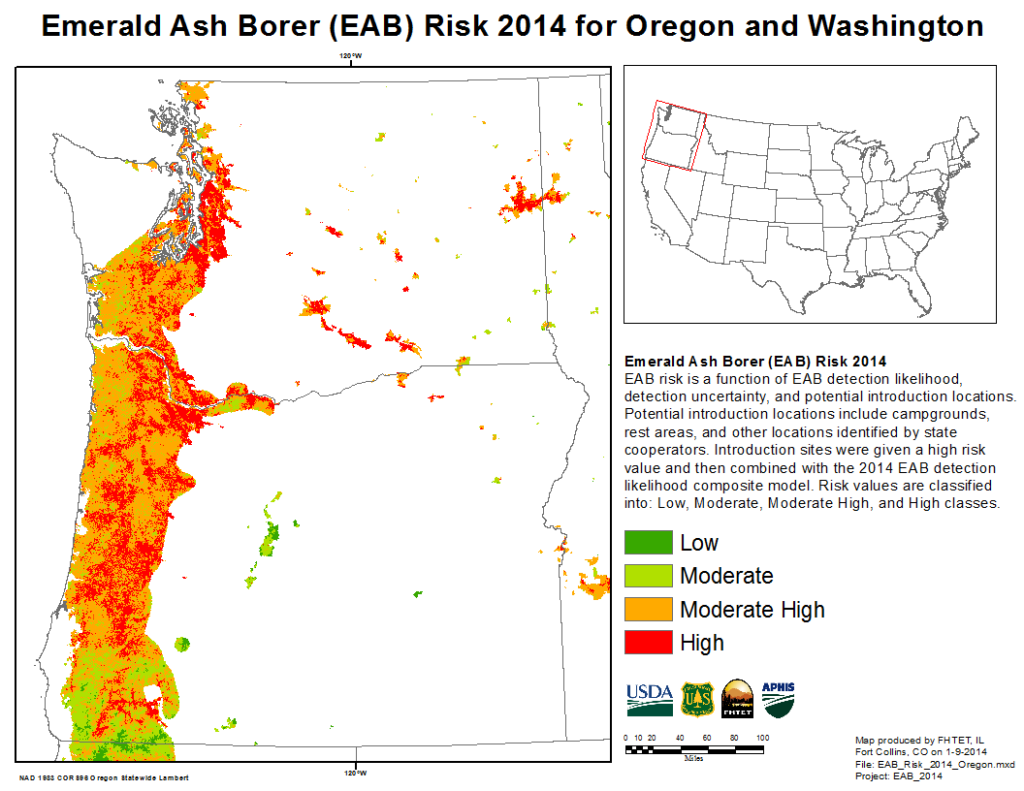
Max Ragozzino of the Oregon Department of Agriculture reported on imminent release of biocontrol agents targetting the recently detected outbreak there. I am encouraged by the rapid response by both the state and APHIS.
EAB resistance in ash
Jennifer Koch (USFS) said the goal is not to produce populations where every seedling is fully EAB-resistant, but to develop populations of ash trees with enough resistance to allow continued improvement through natural selection while retaining sufficient genetic diversity to adapt to future stressors (changing climate, pests, diseases). The program has developed methods to quantify resistance in individuals.. Initial field selections of “lingering ash” were shown to be able to kill as many as 45 % of EAB larvae. Already green ash seedling families have been produced by breeding lingering ash parents. This first generation of progeny had higher levels of resistance, on average, than the parent trees. Each generation of breeding can increase the proportion of resistance. Although the bioassays to test for EAB-resistance are destructive (e.g., cutting and peeling to count numbers of surviving larvae), the potted ash seedling stumps can resprout. Once the new sprouts are big enough they are planted in field trials to correlate bioassay results with field performers. Poor performers are culled; those with higher levels of resistance remain and become sources of improved seed.
To ensure preservation of local adaptive traits, this process must be repeated with new genotypes to develop many seed orchards from across the species’ wide range. To support this work, concerned scientists are building multi-partner collaborative breeding networks. These organizations provide ways for citizens and a variety of partners to engage through monitoring and reporting lingering ash, making land available for test planting, and helping with the work of propagation.
See Great Lakes Basin Forest Health Collaborative » Holden Forests & Gardens (holdenfg.org), Monitoring and Managing Ash (MaMA) – A citizen-science-driven program for conservation and mitigation (monitoringash.org), and TreeSnap – Help Our Nation’s Trees! for more information.
Resistance levels in some of the first generation progeny were high enough for use in horticulture, where it is important that trees can remain healthy in challenging environments (street trees, city parks, landscaping, etc.). Koch hopes to develop about a dozen cultivars comprising the best-performing trees, appropriate for planting in parts of Ohio, Michigan, Indiana, and Pennsylvania. Local NGO partners are planting some of these promising genotypes in Detroit to see how they withstand EAB attack.
The threat to black ash is especially severe, and this species presents unique difficulties. While scientists found several seedlings from unselected seedlots had killed high levels of larvae, those deaths did not always result in better tree survival. Koch thinks the tree’s defense response becomes detrimental to tree by blocking transport of water and nutrients. She is working with experts in genomics and others, such as Kew Royal Botanic Gardens, to try to identify candidate trees for breeding programs. The genomics work has been supported by APHIS and the UK forest research agency, DEFRA. Michigan and Pennsylvania have supported the breeding work. USFS Forest Health Protection has supported work with black and Oregon ash (see below) (J. Koch, USFS, pers. comm.).
Koch has also begun working with Oregon ash, in collaboration with the USFS Dorena Genetic Resource Center (located in Cottage Grove, Oregon) and other partners.
Hemlock woolly adelgid
Scientists are still trying to find the right combination of biocontrol, chemical treatments, and silvicultural manipulation.
For several years, hope has focused on two has been on two predatory beetles, Laricobius nigrinus and L. osakiensis. Scott Salom (Virginia Tech) reports that release of these beetles over the past 20 years has had a significant impact on HWA density and tree photosynthetic rate and growth. However, Laricobius aredifficult to rear and they attack only the sistens generation of the adelgid. Ryan Crandall (University of Massachusetts) reports it has been difficult to establish these beetles in the Northeast. He links this difficulty is caused by temporary drops in HWA populations after cold snaps.
Scientists now agree that need to find predators that attack HWA during other parts of its lifecycle. Hope now focuses on silverflies — Leucotaraxis argenticollis and Le. piniperda. While both species are established in eastern North America, the clades in the east feed almost exclusively on pine bark adelgid, and have not begun attacking HWA. Biocontrol practitioners therefore collect flies in the Pacific Northwest for release in the east. Salom is increasing his lab’s capacity to rear silverflies and exploring release strategies.
Preliminary evidence indicates that the western clades of Leucotaraxis are establishing, although data are not yet definitive (Havill, USFS).
Detecting the presence of biocontrol agents presents several challenges. Tonya Bittner (Cornell) described efforts to use eDNA analysis for this. Some puzzles have persisted; e.g., at some sites, she detected eDNA but caught no silverflies. This raised the question of long eDNA associated with the original release might persist. Another problem is that the assay cannot separate the introduced western L. nigrinus from the native congener, L. rubus (which also does not feed on HWA). She continues efforts to improve this technique.
Others explored interactions of the biocontrol agents with insecticides. Salom is studying the impact of soil-applied insecticides on Laricobius populations, which aestivate in the soil. Preliminary results showed significant reduction in the beetle’s population under soil drench application but not under soil injection. He has not yet analyzed all the data.
Michigan is trying to prevent spread of HWA from five counties along the eastern shore of Lake Michigan (where HWA was introduced on nursery stock) to widespread hemlock forests in northern part of the state. Phil Lewis (APHIS) is studying persistence of systemic insecticides in hemlock tissues, particularly twigs and needles. The pesticides involved are imidacloprid, dinotefuran, and Olefin. He has found that pesticide levels are highest 18 – 22 months after treatment, then decline. They are significantly higher after trunk injection compared to bark spray or soil treatments. Imidacloprid had higher residues in twigs; dinotefuran in needles. This difference affects the likelihood of adelgids actually ingesting the toxin.
Bud Mayfield (USFS) reported on his study of silvicultural strategies to support healthier hemlocks. While hemlocks normally thrive in shade, it has been determined that sunlight assists small trees reducing HWA sufficiently to counter the tree’s leaf-level stress. Small sapling hemlocks grown in sunlight fix more carbon and convert it to growth in shoots and trunk diameter.
Mayfield found promising immediate suppression of HWA in large gaps in Georgia and Tennessee. By the third year the saplings were still growing, although their faster growth had attracted more HWA. These findings were less clear farther north in central Virginia and western Maryland – Mayfield thinks because HWA pressure there is lower. However, managers must maintain the gaps by cutting rapidly-growing competing woody species. He plans to test this strategy farther north in Pennsylvania. He is still trying to determine the optimal size of the gap.
Posted by Faith Campbell
We welcome comments that supplement or correct factual information, suggest new approaches, or promote thoughtful consideration. We post comments that disagree with us — but not those we judge to be not civil or inflammatory.
For a detailed discussion of the policies and practices that have allowed these pests to enter and spread – and that do not promote effective restoration strategies – review the Fading Forests report at http://treeimprovement.utk.edu/FadingForests.htm
or